Pipeline
CU101
Pipeline
CU101
CU102
Expanded indications of CU06 to Acute Lung Injury
| Sortation | content |
|---|---|
| CU102 | Expanded indications of CU06 to Acute Lung Injury |
| Indication | Acute Lung Injury |
| Unmet Needs | Glucocorticoids, inhaled nitric oxide, and activated protein C (APC) have been tried as therapeutic agents, but they have low clinical efficacy and side effects. |
| Mechanism of Action |
Mediates endothelial cell homeostasis and blocks vascular leakage and inflammation caused by various vascular endothelial activators. |
| Efficacy & Safety |
• The first in class endothelial dysfunction blocker for acute lung injury • Inhibits excessive inflammatory reaction in the early (acute) stage. • Suppresses the proliferation of alveolar epithelial cells in the intermediate (proliferative) stage. • Restores endothelial barrier destroyed by mediators (IL-6, IL-1β, TNF-α). |
| Market |
• USD 1579 million in 2020 • USD 2417 million by 2030 (lung injury / CAGR: 4.2%) |
Indication
Cause
Incidence, Mortality
Symptoms
Unmet Needs
Glucocorticoids, inhaled nitric oxide, and activated protein C (APC) have been tried as therapeutic agents, but they have low clinical efficacy and side effects.
Mechanism of Action
Pathogenesis of ALI
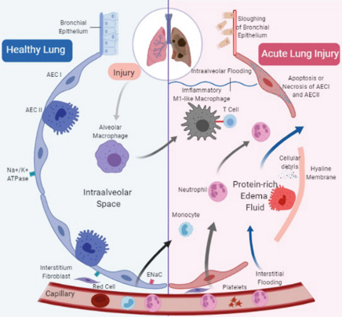
Damages on vascular endothelium and alveolar epithelium
Lung edema & Inflammation
Acute Lung Injury
Blocking blood vessel leakage and inflammation emerged as a treatment option
CU102: principle of action
Lungs in COVID-19 patients: vascular endothelial dysfunction observed as a key finding
CU102 blocks
The function of CU102 to inhibit endothelial cell-specific apoptosis, block blood vessel leakage and inflammation is noted as a novel treatment for ALI
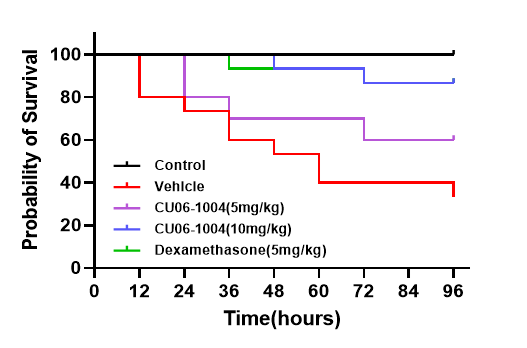
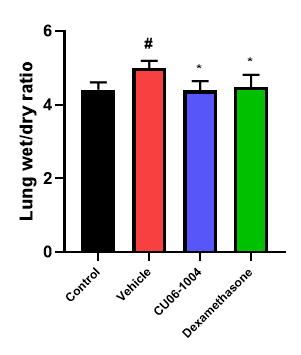
▶ Proven excellent efficacy in ALI animal models
Efficacy
Improvement of survival rate & inhibition of pulmonary edema
Oral administration of CU102 (10mg/kg) showed comparable survival rate to dexamethasone (steroid) and also relieved pulmonary edema
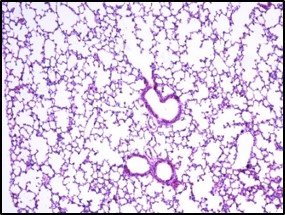
Inhibition of inflammation in lung tissue
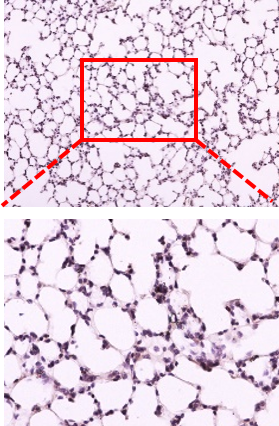
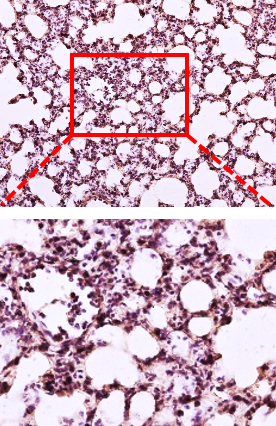
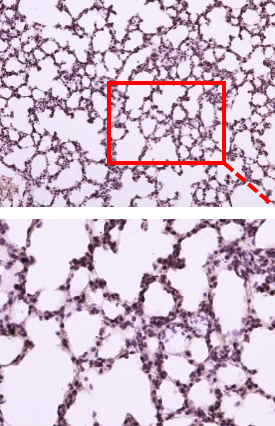
Reduces the influx of inflammatory cells into the lung tissue and tissue damage caused by LPS
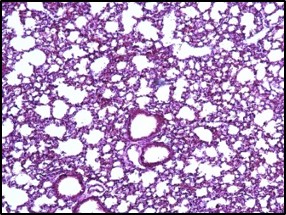
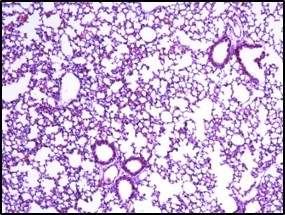
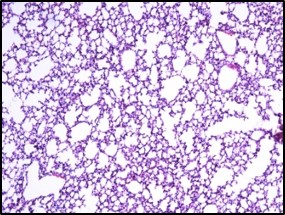
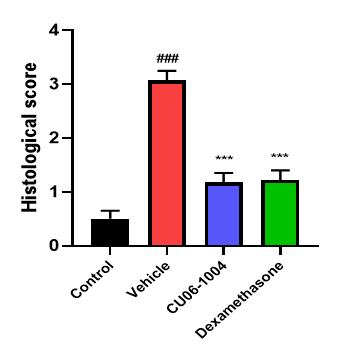
Electron microscope data of alveoli and pulmonary blood vessels
CU102 improves the alveolar-capillary barrier thickened by edema after LPS administration and significantly inhibits the adhesion of inflammatory cells to the inside of the pulmonary blood vessels
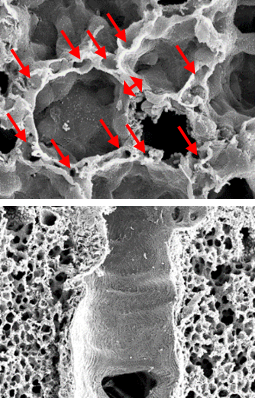
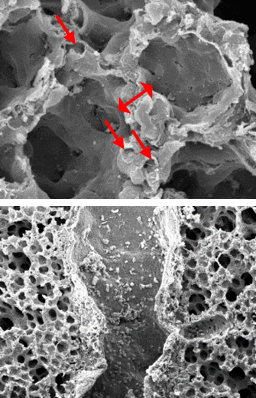
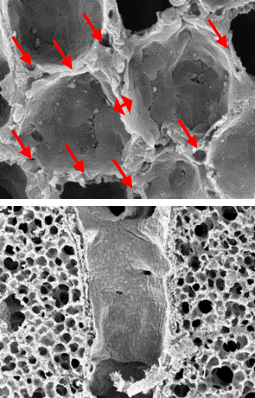
Arrow: normal capillary, Two-headed arrow: alveolar wall thickness
The structure of alveolar capillary endothelial cells damaged by LPS is improved
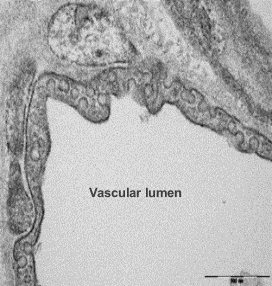
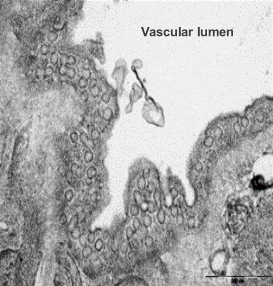
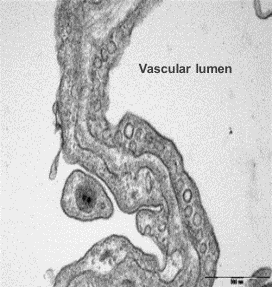
Additional Research
CU102 significantly inhibits MPO activity
Myeloperoxidase (MPO) is a peroxidase enzyme most abundantly expressed in neutrophil granulocytes
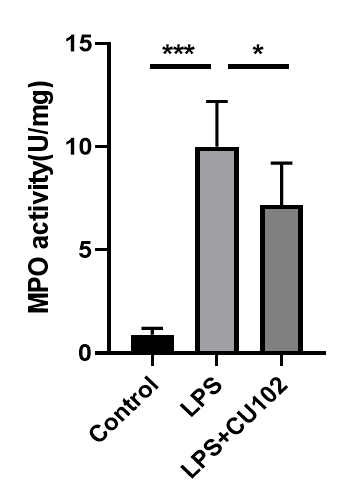
Cytokine Analysis in biological samples
BALF (Bronchoalveolar lavage fluid)
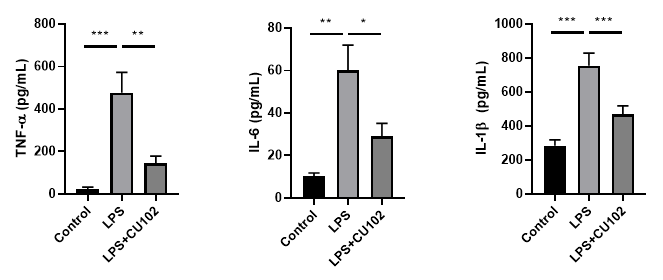
Serum
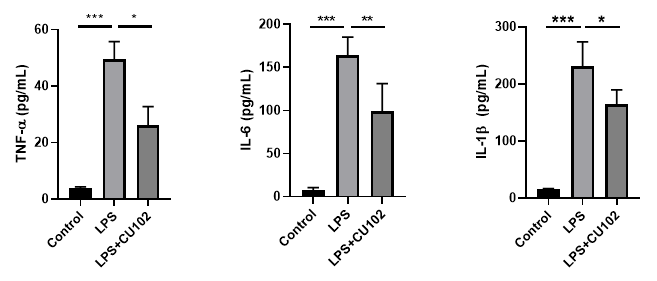
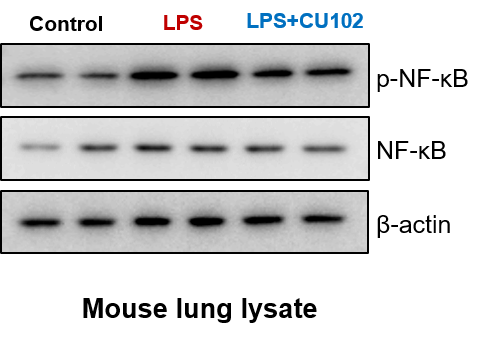
Additional Research
Verified CU102’s suppression of NF-ĸB expression and activation using immunohistochemical staining and Western blot.